COVID-19 Vaccines
Sharon Moise, MD, and Mayuri Bhakta, FNP-BC
December 3, 2020
The COVID-19 pandemic was caused by the SARS-CoV-2, a virus first identified in December 2019 in Wuhan, China. The respiratory disease rapidly spread worldwide and has impacted global health, economy, education, and virtually every facet of society. Research has been taking place worldwide to better understand the SARS-CoV-2 virus, COVID-19 disease process, treatment options and measures help to prevent the spread of disease, including vaccines.
COVID-19 Vaccine Development
To date, in the United States alone, there have been over 12 million cases of COVID-19 and over 250,000 COVID-19 related deaths. Researchers and multiple pharmaceutical companies have been racing to produce a safe and effective COVID-19 vaccine.
A vaccine is a biological substance that stimulates a person’s immune system to produce immunity to a specific disease, so that the person can be exposed in the future without becoming infected. A vaccine typically contains particles that resemble a disease-causing microorganism. These particles are typically made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins (antigens).
The particles “teach” the body’s immune system to recognize the virus as a threat and to destroy it. Additionally, the experience of the vaccine helps the body to remember and destroy the virus, in the event the person encounters the virus in the future.
Since the start of the COVID-19 pandemic, tremendous worldwide scientific research has led to the development of many different types of vaccines. The World Health Organization (WHO) has compiled a comprehensive list of vaccines, their manufacturers, vaccine types and clinical trial status.
Historically, the two types of vaccines that have been most commonly used are whole pathogen vaccines and subunit vaccines:
- Whole pathogen vaccines: These vaccines use an entire virus that has been either killed (inactive) or very weakened viruses (attenuated). Examples of whole pathogen vaccines include the flu shot (inactive) and measles, mumps, rubella (MMR) vaccine (attenuated). There are currently no manufacturers using this modality to develop COVID-19 vaccines.
- Subunit vaccines:Rather than using the entire virus (like whole pathogen vaccines), manufacturers of subunit vaccines use a small signature protein subunit of the virus (antigen). In the case of COVID-19, the signature protein subunit selected was the tiny spikes seen on the outside of the COVID-19 virus. While the spike protein subunit was a good target to use, researchers found that individual spikes were too small to elicit the necessary immune response. As such, vaccine developers can add other proteins to the vaccine or combine many of the spike subunits together to make a larger “ball” so that the body identifies the particles as a threat and elicits the needed immune response. Examples of subunit vaccines include: Hepatitis B, pneumonia, and shingles vaccines. Of note, several subunit COVID-19 vaccines are in different phases of clinical trials.
The next generation in vaccine development is represented in mRNA vaccines (nucleic acid vaccines). These vaccines have previously made it to human trials for the prevention of other diseases, but none have been FDA approved. Moderna and Pfizer, in partnership with BioNTech, have both developed mRNA vaccines for the prevention of COVID-19. The Moderna and Pfizer mRNA COVID-19 vaccines are the first vaccines of their kind to apply for an Emergency Use Authorization (EUA) by the FDA. What makes mRNA vaccines different?
- In mRNA vaccines, a vaccinated person receives genetic material, mRNA, that encodes for a specific viral protein. When the mRNA is injected into the arm, cells in the injected person start producing antigen subunits (the spike proteins). These antigen subunits provide the immune system with a preview of what the real virus looks like without causing COVID-19. The body responds to the preview of the antigen (spike protein) by creating both antibodies and memory B cells. The antibodies and memory B cells stay in the body so that if the person has an exposure to the virus, the body is prepared and can destroy the virus so that the person does not become infected.
Operation Warp Speed and Emergency Use Authorization (EUA) Approval
Operation Warp Speed is an interagency, public-private partnership initiated by the U.S. government in early April 2020 to facilitate and accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics. The CARES Act provided Operation Warp Speed with almost 10 billion dollars to expedite vaccine research and development.
Typically, medications, vaccines, and devices get FDA approval after they have gone through an intensive, and frequently time-consuming, investigation. The EUA process is different than an FDA approval or clearance process. Under the EUA process, in emergency situations when there are no adequate, approved and available alternatives, the FDA has the authority to authorize medical products for use under specified conditions before all the evidence that would be needed for full FDA approval is available. In considering EUA requests, the agency promptly and carefully evaluates the totality of the scientific evidence that is available on the product’s safety and effectiveness to determine whether the medical product may be effective for its proposed authorized uses.
The EUA approval for a COVID-19 vaccine requires:
- A minimum of 3,000 participants that are vaccinated
- 2 months of observation and evaluation post-vaccination
- At least a 50% efficacy rate
The Pfizer and Moderna mRNA COVID-19 Vaccines
Pfizer was the first COVID-19 vaccine developer to apply for an FDA EUA. Pfizer had a large-scale phase 3 clinical trial that included 42,538 participants. The Pfizer vaccine data was submitted to the independent data monitoring company once 94 participants tested positive for COVID-19. The independent data monitoring company determined the vaccine was 95% effective. The vaccine requires two vaccine doses 3–4 weeks apart. The Pfizer COVID-19 mRNA vaccine will reportedly need to be stored at about -70°C (-94°F) during transport and will remain stable for up to six months if stored at that temperature. It can be stored in freezer containers with dry ice for up to 5 days. While Pfizer has announced that it will be conducting further studies to determine the viability of the vaccine at higher temperatures, based on current cold temperature requirements, the vaccine will require an industrial grade ultra-low temperature (ULT) freezer to remain stable. This type of freezer can be costly and require additional safety measures.
The Moderna COVID-19 vaccine also had a robust phase 3 clinical trial that included 30,000 participants. Much like the Pfizer vaccine, the Moderna COVID-19 mRNA vaccines require two vaccinations spaced 3–4 weeks apart. The Moderna vaccine data was submitted to an independent data monitoring company once 95 participants tested positive for COVID-19. The independent data monitoring company determined the vaccine was 94.1% efficacy. According to a recent press release, Moderna announced new data showing that its COVID-19 vaccine remains stable at 2° to 8°C (36° to 46°F), the temperature of a standard home or medical refrigerator, for 30 days. Moderna announced the vaccine would remain stable at -20°C (-4°F) for up to six months, at refrigerated conditions for up to 30 days and at room temperature for up to 12 hours.
Side effects from the Moderna and Pfizer vaccines have not been published; however, side effects with other vaccinations may include: fever, pain and soreness at the injection site, chills, mild flu-like illness, headaches, and body aches.
Distribution of Vaccines
The Advisory Committee on Immunization Practices (ACIP) will give recommendations for the distribution of vaccines based on risk factors of population groups. Based on this information, we can speculate that there will be a multi-phased approach to vaccination.
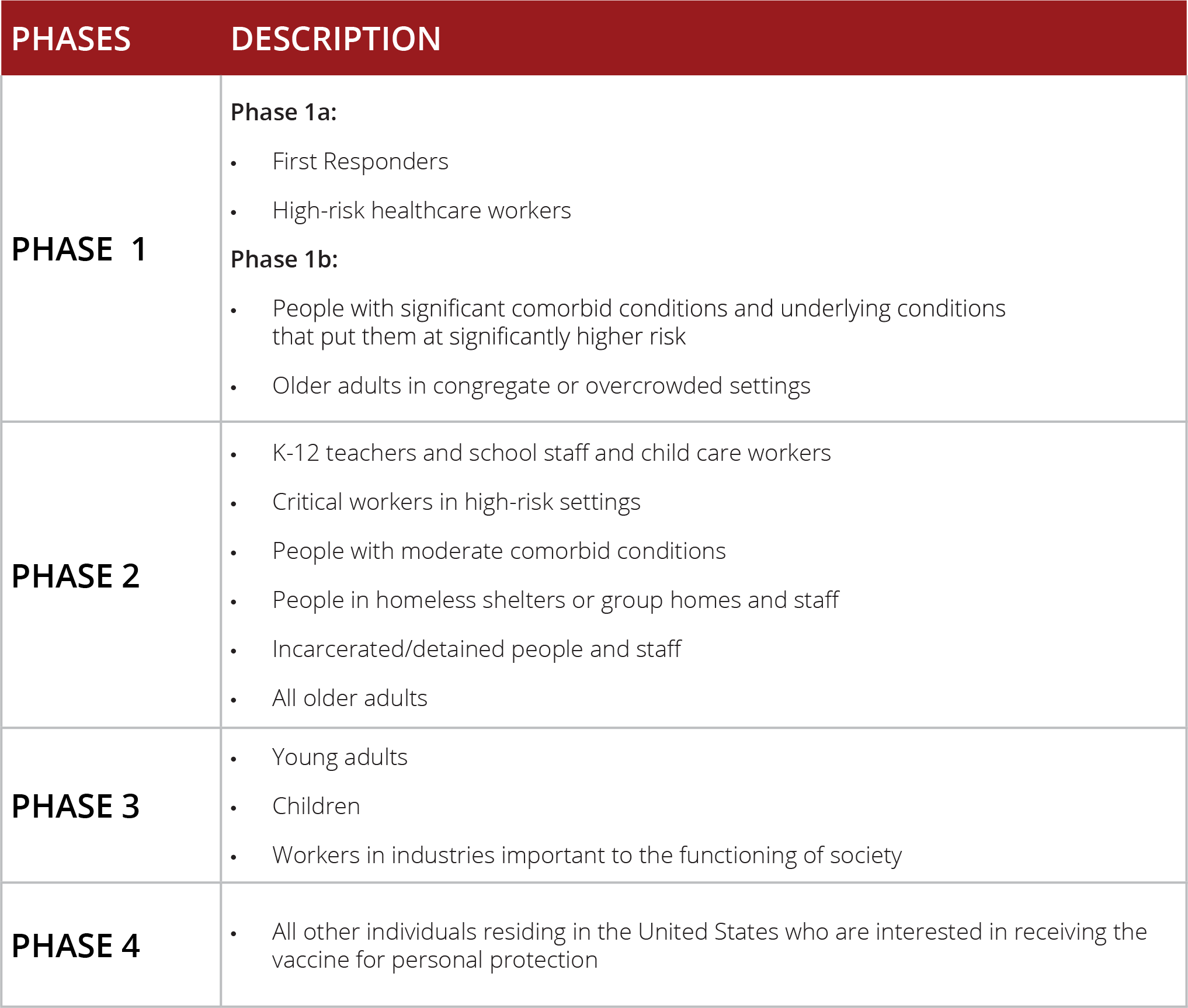
Phase 1 may include at-risk health workers and first responders, then people living in skilled nursing facilities and people with comorbid conditions that put them at very high risk.
Phase 2 may include schoolteachers, critical workers in high-risk settings, people with comorbid conditions that put them at moderate risk, all older adults, and people living in homeless shelters or group homes.
Phase 3 and 4 may include young adults, children, and the rest of the U.S. population.
Challenges
The two COVID-19 vaccine candidates at the forefront of the race for a COVID-19 vaccine have shown good effectiveness, based on initial data. The CDC and state health departments will face some challenges with both. First will be the availability of the vaccine in 2020 into 2021. The second will be the logistics surrounding transportation, storage, and administration of the vaccines.
Both the Pfizer and Moderna vaccines are mRNA vaccines, which tend to be more unstable and degrade into smaller subunits at warmer temperatures. The Pfizer vaccine requires transport and storage at sub-freezing temperatures, temperatures found in cryofreezers.
Based on recent press releases, Moderna seems to be more promising in regard to transportation and storage of the vaccine. According to Moderna, the vaccine can be stored in a regular freezer for up to 6 months and can be refrigerated for up to 30 days. If the Pfizer vaccine needs to be stored for use beyond 5 days, a special freezer (cryofreezer) will be required. At this time, the Pfizer vaccine will require special packaging with dry ice and a navigator chip to monitor the location and temperature of the vaccines.
Every effort will need to be made to coordinate vaccinations. Vaccinations in rural areas may present more coordination and may present more difficulties for storage and timely administration.
The other challenge will be scheduling and administering the second vaccine within the 3–4 week anticipated timeframe. This will be a large undertaking for all involved in the vaccination process. All systems administering the vaccine will need to have processes in place for recall and scheduling of patients for their second vaccination. All efforts will need to be made to avoid waste of vaccines that will, at least initially, be in a limited supply.
CDC’s COVID-19 Distribution Planning
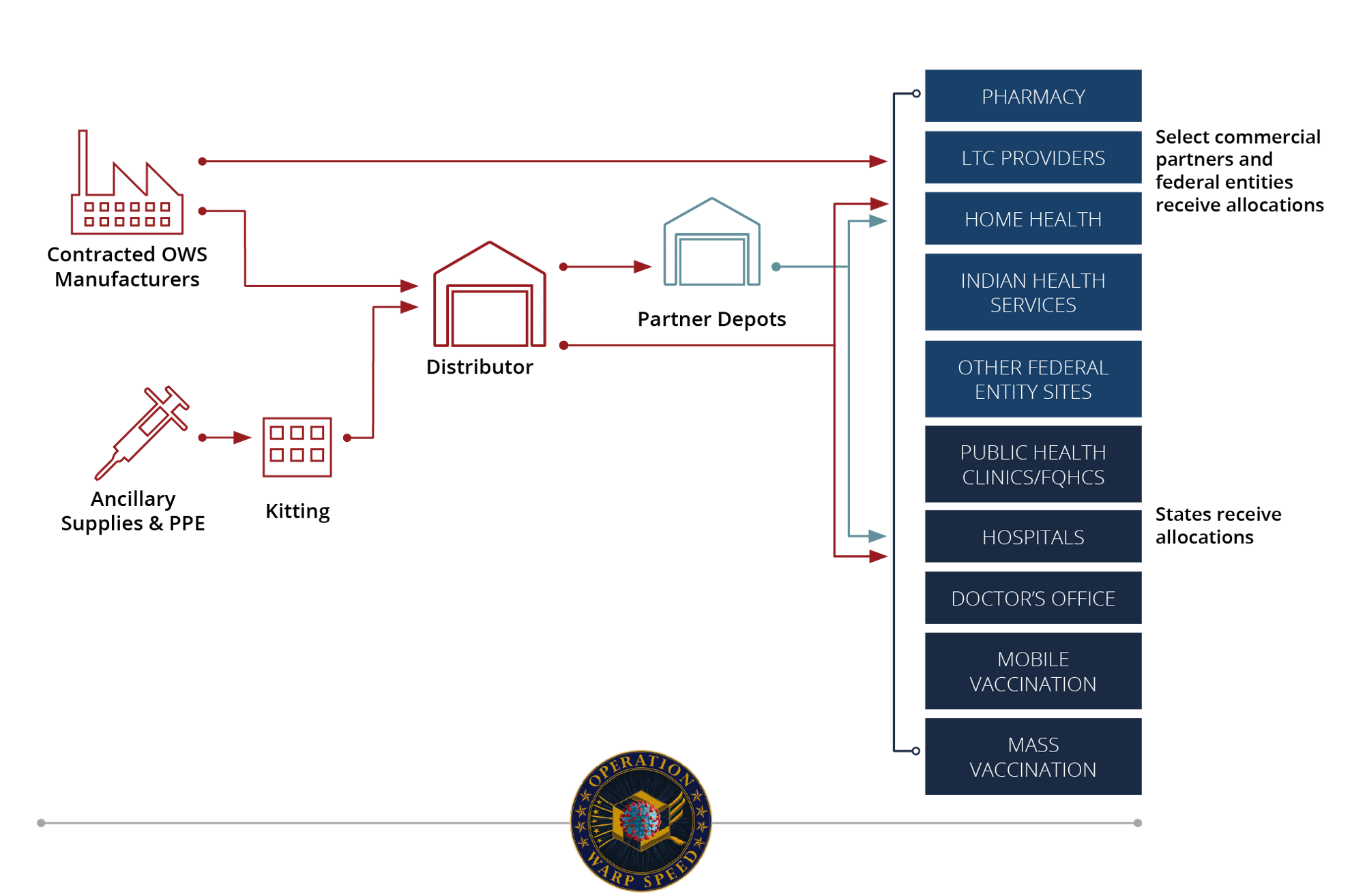
According to the Operation Warp Speed (OWS) Strategy for Distributing a COVID-19 Vaccine, the primary objective of OWS is to ensure that every American who wants a COVID-19 vaccine can receive one, starting January 2021. OWS is partnering with experts from the Department of Defense (DoD) and the Centers for Disease Control (CDC) and the Department of Health and Human Services (HHS) to coordinate supply, production, and distribution of vaccines. Eligible COVID-19 vaccines will be arranged for delivery according to distribution plans after FDA authorization. Distribution efforts should ensure safety of the products, maintain and control visibility, ensure traceability of the product and maximize coverage.
Successful implementation of a national COVID-19 vaccination program will require coordination across federal, state, local, tribal, and territorial governments along with public and private partners. Distribution plans developed by OWS have three components:
- Partnerships with state, local and tribal health departments, territories, and federal entities to allocate and distribute vaccines, augmented by direct distribution to commercial partners.
- A centralized distributor contract with potential for back-up distributors for additional storage and handling requirements.
- A flexible, scalable, secure web-based IT vaccine tracking system for ongoing vaccine allocation, ordering, uptake, and management.
The distribution plan calls for centralized distribution of COVID-19 vaccines which will allow the government full visibility, control and the ability to reallocate vaccines as needed to optimize vaccine use. On August 14, the CDC awarded McKesson with a centralized distributor contract for future COVID-19 vaccines that are refrigerated (2–8°C) or frozen (-20°C). Vaccines cannot be ordered from McKesson; interested parties must contact state, local or tribal health departments or the CDC.
Ultra-frozen (-70°C and colder) vaccines are not within the scope of McKesson’s contract with the CDC. It has been reported that Pfizer will be its own distributor of vaccine as Pfizer has developed their own “thermal shipper.”
Vaccines will be distributed to point-of-care facilities. Vaccines will be administered along with ancillary supplies and PPE in “kits.” The difficult work of allocating the limited initial supplies of a vaccine will fall on state, local, and tribal health departments. Each state has been required to submit a COVID-19 vaccine plan. Each state’s plan should address to which point-of-care facilities the vaccines will be initially distributed. Select commercial partners and federal entities will receive initial allocations of limited supplies of vaccine. These include select chain and community pharmacies (e.g. CVS, Walgreens, Albertsons, etc.), long-term care providers, home health, Indian health services, and other federal entity sites. Availability of COVID-19 vaccines to other clinics will be based on state-based allocation. This will likely occur once more vaccine is available for distribution.
Overview of Distribution and Administration
Operation Warp Speed, defense.gov
The CDC has published a COVID-19 Vaccination Support Page which outlines data and reporting, vaccine data requirements, documentation, and operational guidance.
Phased Approach to Vaccination
As of November 30, 2020, both Pfizer and Moderna have applied for an FDA EUA for their COVID-19 vaccines. Even if both vaccines receive an EUA, doses will be limited. The National Academies formed a consensus study to assist in planning for an unbiased distribution of COVID-19 vaccines.
The recommendations for vaccine distribution are evolving on a weekly basis. At this time, based on the consensus study, the current recommendation for vaccine allocation and distribution is divided into four phases.
Ongoing Developments
Vaccines, vaccine development and vaccine distribution will all continue to be an evolving topic as new vaccines are approved and compared.
References
Centers for Disease Control and Prevention, "10 Things Healthcare Professionals Need to Know about U.S. COVID-19 Vaccination Plans," November 20, 2020, https://www.cdc.gov/coronavirus/2019-ncov/hcp/vaccination.html
Centers for Disease Control and Prevention, "COVID-19 Vaccination Provider Support," November 13, 2020, https://www.cdc.gov/vaccines/covid-19/vaccination-provider-support.html
Centers for Disease Control and Prevention, "The COVID-19 Vaccination Program Interim Operational Guidance for Jurisdictions Playbook," November 5, 2020, https://www.cdc.gov/vaccines/covid-19/covid19-vaccination-guidance.html
Damian McNamara, "Moderna COVID-19 Vaccine: Interim Data Show 94.5% Efficacy," Medscape, November 16, 2020, https://www.medscape.com/viewarticle/941023
Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals," https://pfe-pfizercom-d8-prod.s3.amazonaws.com/2020-11/C4591001_Clinical_Protocol_Nov2020.pdf
McKesson, "McKesson to distribute future COVID-19 vaccines in support of Operation Warp Speed," August 2020, https://www.mckesson.com/About-McKesson/COVID-19/Vaccine-Support/
Moderna, "Moderna Announces Longer Shelf Life for its COVID-19 Vaccine Candidate at Refrigerated Temperatures," November 16, 2020, https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-longer-shelf-life-its-covid-19-vaccine
National Community Pharmacists Association, "COVID-19 Vaccine Information," https://ncpa.org/covid-vaccine
National Institute of Allergy and Infectious Disease, "Vaccine Types," July 1, 2019, https://www.niaid.nih.gov/research/vaccine-types
Pfizer, "Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study," November 9, 2020, https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against
Pfizer, "A Phase 1/2/3 Study to Evaluate the Safety, Tolerability, Immunogenicity, and
Pien Huang, "First COVID-19 Vaccine Doses To Go To Health Workers, Say CDC Advisers," National Public Radio, November 5, 2020, https://www.npr.org/sections/health-shots/2020/11/05/931844298/first-covid-19-vaccine-doses-to-go-to-health-workers-say-cdc-advisers
The National Academies of Sciences, Engineering, and Medicine, "A Framework for Equitable Allocation of Vaccine for the Novel Coronavirus," https://www.nationalacademies.org/our-work/a-framework-for-equitable-allocation-of-vaccine-for-the-novel-coronavirus#sectionProjectScope
The National Academies Press, "Framework for Equitable Allocation of COVID-19 Vaccine," https://www.nap.edu/read/25917/chapter/1
U.S. Department of Health and Human Services, "Trump Administration Partners with Chain and Independent Community Pharmacies to Increase Access to Future COVID-19 Vaccines," November 12, 2020, https://www.hhs.gov/about/news/2020/11/12/trump-administration-partners-chain-independent-community-pharmacies-increase-access-future-covid-19-vaccines.html
U.S. Department of Health and Human Services, "Vaccine Types," March 2020, https://www.vaccines.gov/basics/types
U.S. Food and Drug Administration, " Emergency Use Authorization," November 27, 2020, https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
U.S. Food and Drug Administration, "COVID-19 Update: FDA’s Ongoing Commitment to Transparency for COVID-19 EUAs," November 17, 2020, https://www.fda.gov/news-events/press-announcements/covid-19-update-fdas-ongoing-commitment-transparency-covid-19-euas
U.S. National Library of Medicine, "A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19," October 28, 2020, https://clinicaltrials.gov/ct2/show/study/NCT04470427?term=NCT04470427
World Health Organization, "Draft landscape of COVID-19 candidate vaccines," November 12, 2020, https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines







